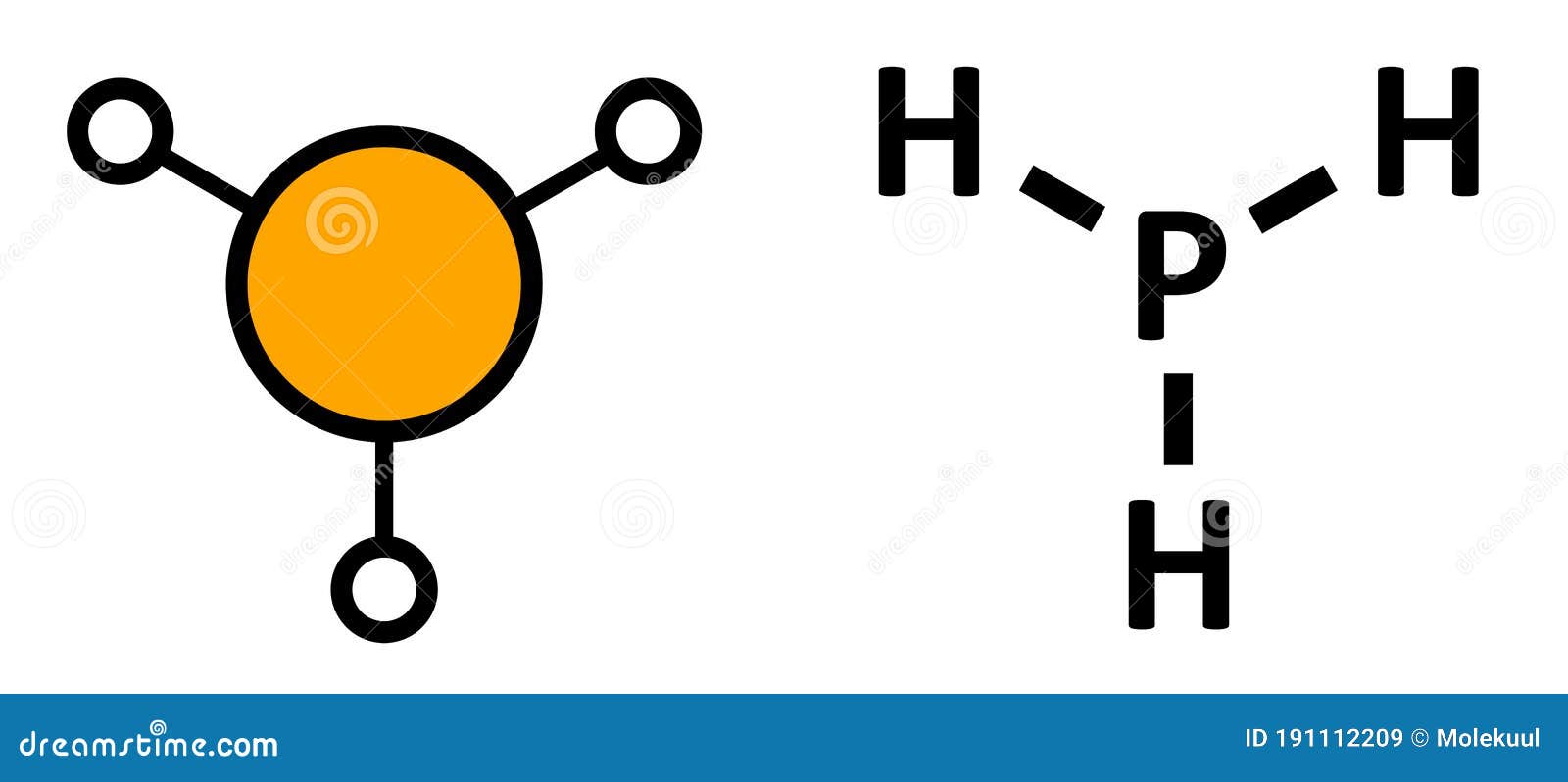
Ph3 Lewis Structure Shape
* Phosphorus requires a full octet of electrons, and brings 5 with it.* Each Hydrogen brings 1 electron* This allows for three single bonds and ONE lone pair.
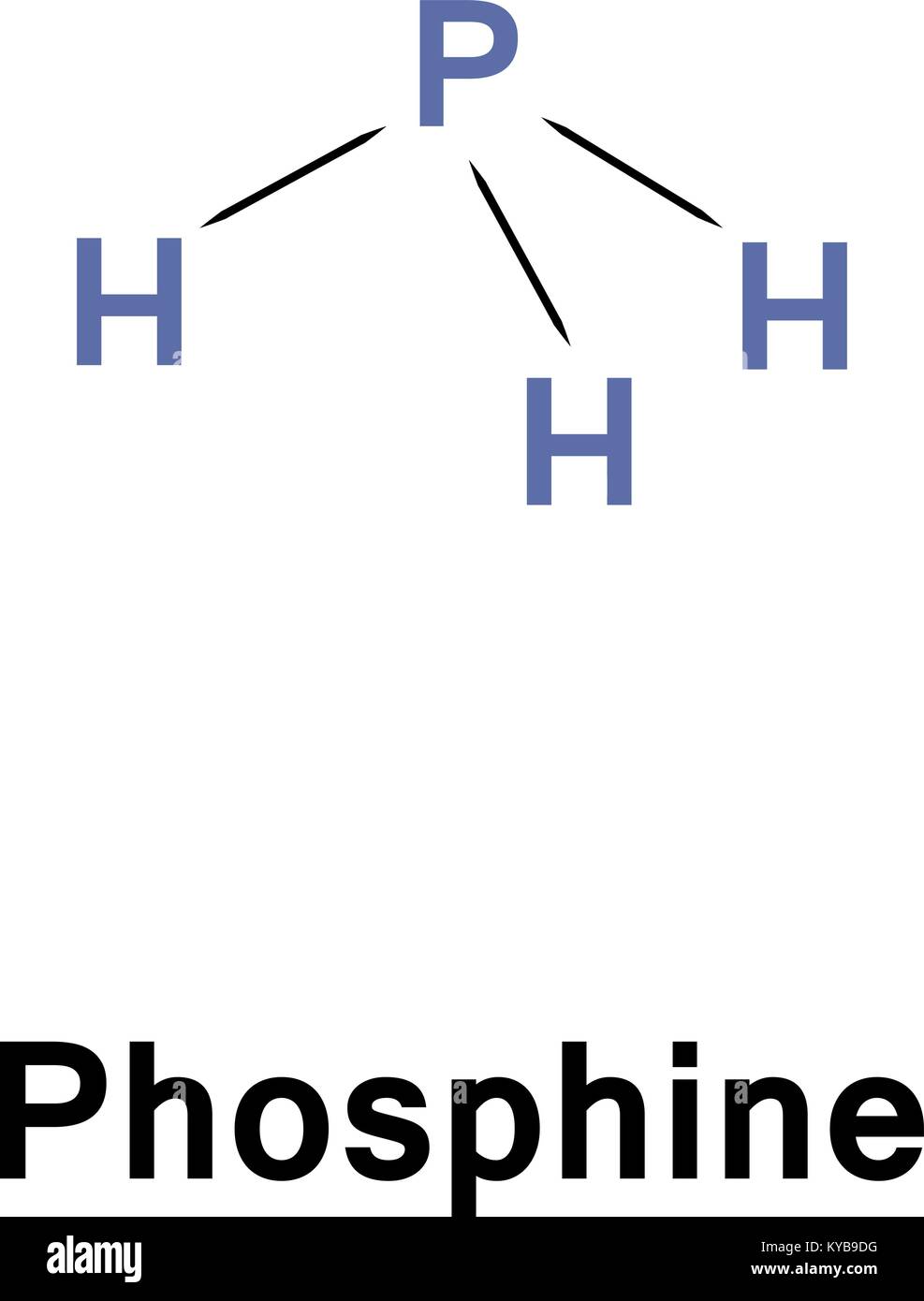
Ph3 Lewis Structure Shape
The Lewis electron structure for the NH 4+ ion is as follows: The nitrogen atom shares four bonding pairs of electrons, and a neutral nitrogen atom has five valence electrons. Using Equation 4.4.1, the formal charge on the nitrogen atom is therefore. formalcharge (N)=5− (0+82)=0 .
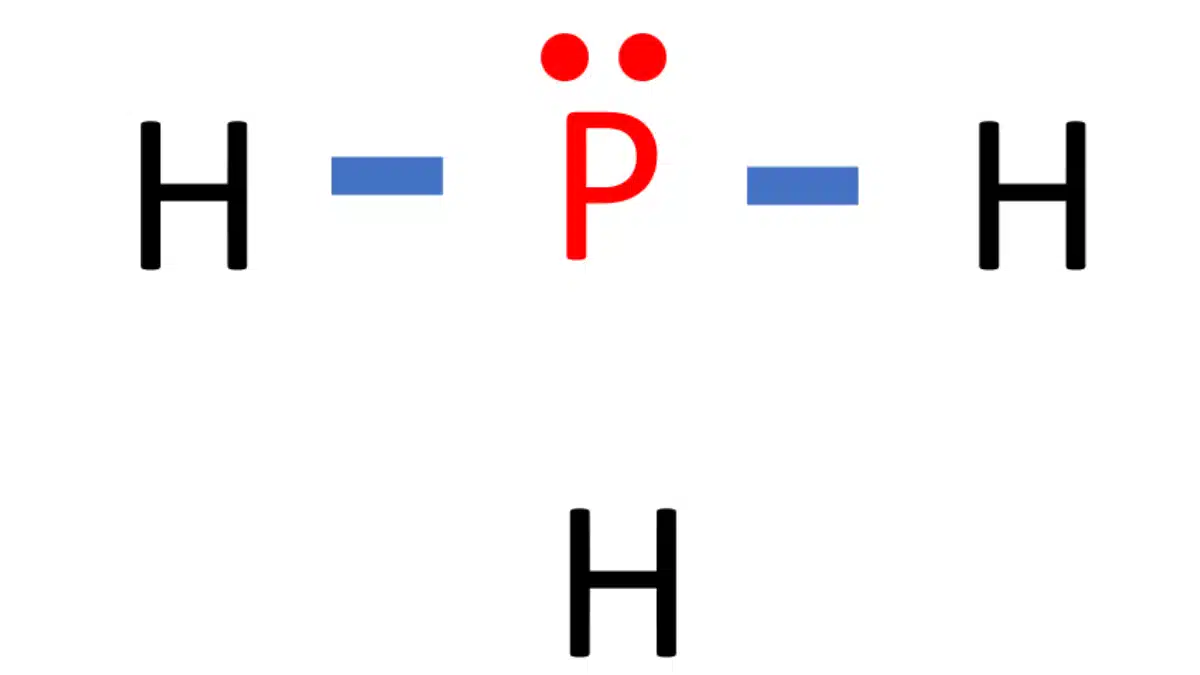
PH3 Lewis Structure in four simple steps What's Insight
This widget gets the Lewis structure of chemical compounds. Send feedback | Visit Wolfram|Alpha Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.

Draw The Lewis Structure Of Ph3
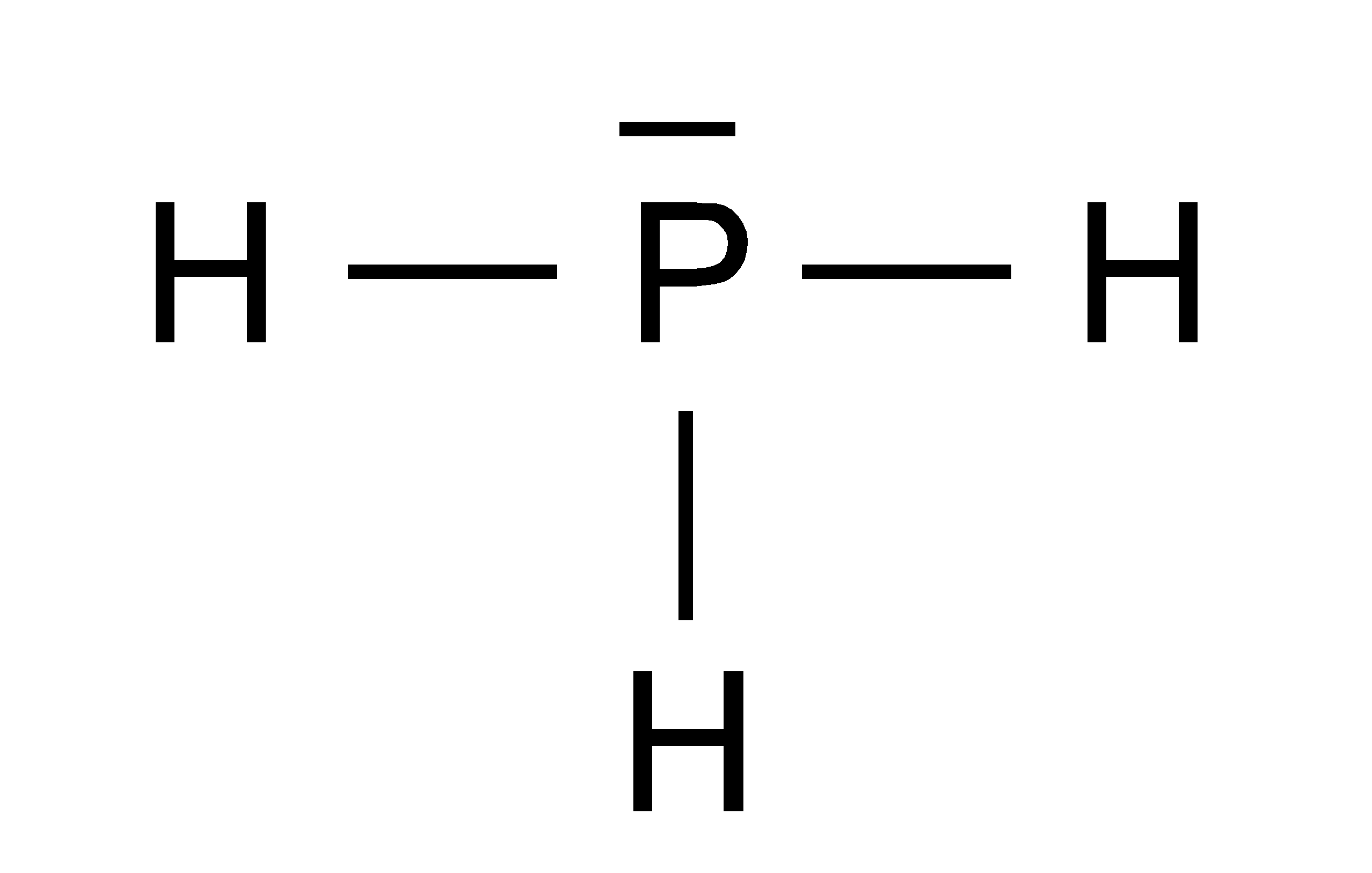
PH3 lewis structure has a Phosphorus atom (P) at the center which is surrounded by three Hydrogen atoms (H). There are 3 single bonds between the Phosphorus atom (P) and each Hydrogen atom (H). There is 1 lone pair on the Phosphorus atom (P).

PH3 Lewis Structure How to Draw the Lewis Structure for PH3 YouTube
PH3 or phosphine is a compound of phosphorus that is classified under pnictogen hydride. Phosphine or phosphane forms bonds by covalent bonding. We can study the bonding in the molecule of PH3 by taking into consideration lewis method. We will study the PH3 lewis structure and understand the concept. Some facts about Phosphane

How to draw PH3 Lewis Structure? 4
We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of.
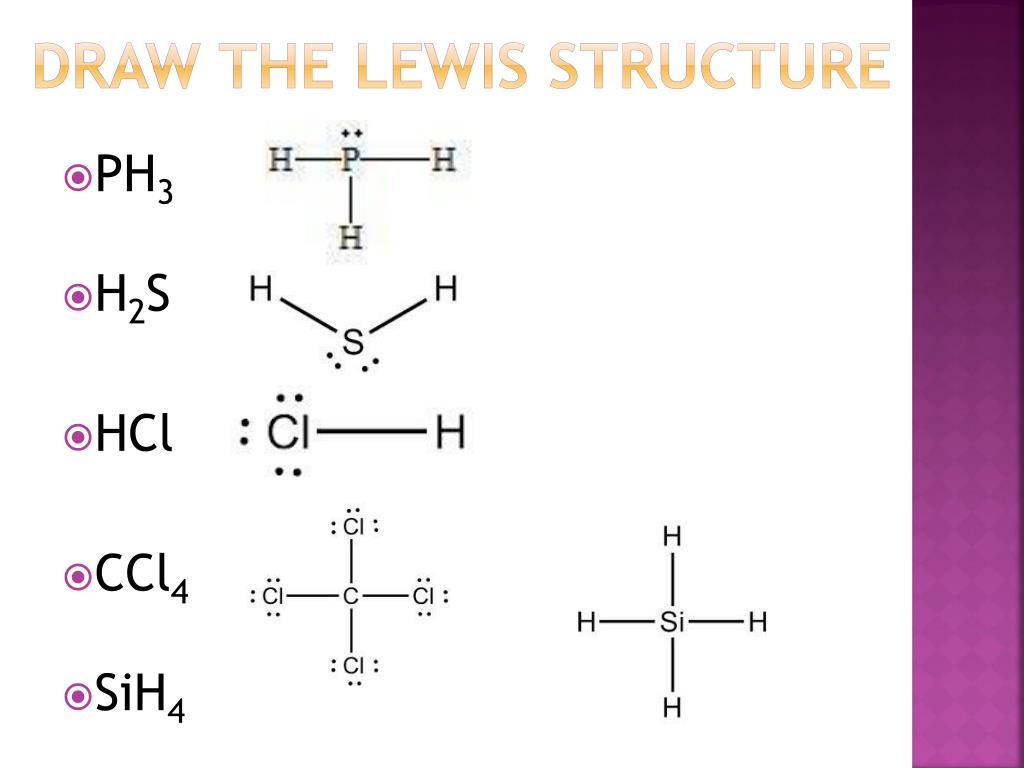
PPT Covalent Bonds PowerPoint Presentation, free download ID3048466
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

Ph3 Lewis Structure Shape
This tutorial shows you how to create the Lewis structure and moleculargeometry for phosphine (PH3).

Ph3 Lewis Structure Shape
Phosphine PH3 Lewis Dot Structure shadowboy220 1.9K subscribers 49K views 11 years ago Chemistry Lewis Dot Structures A video explanation of how to draw the Lewis Dot Structure for.

Step2 Lewis Structure of PH3 for constructing around the central
In phosphine (PH 3) lewis structure, there are three sigma bonds and one lone-pair around phosphorous atom. No charges on phosphorous atom and hydrogen atoms. Shape of PH 3 is trigonal pyramidal. Molecular geometry around phosphorous atom is tetrahedral. Total valence electrons pairs around phosphorous atom is four.
Ph3 Lewis Structure Shape
The total valence electron available for drawing the Phosphine (PH3) Lewis structure is 8. There is no hybridization occurring in PH 3, because, it is a Drago molecule. We can also say that PH 3 has "Zero" or "Nil" hybridization. The H-P-H bond angle in PH 3 is 93.5º.

Ph3 Lewis Structure Shape
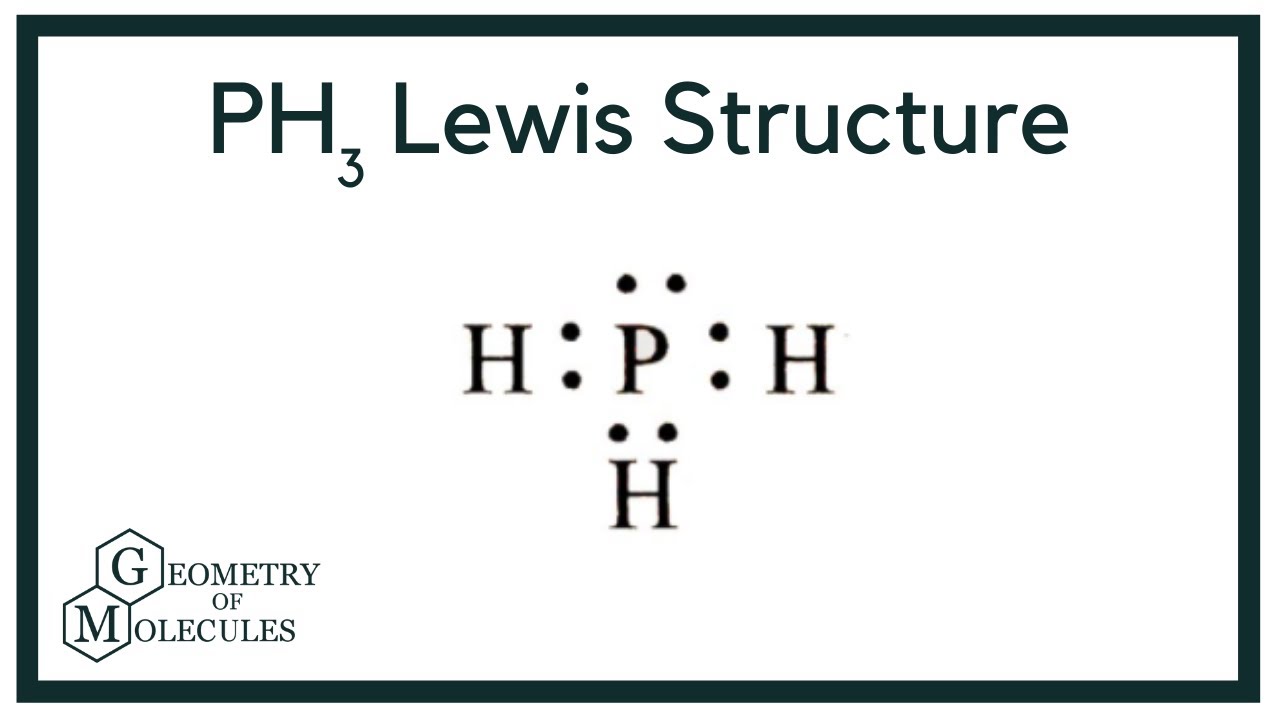
In the Lewis structure of PH3 structure there are a total of 8 valence electrons. PH3 is also called Phosphine. The Lewis structure for PH3 is similar the the structure for NH3 since.

Intermolecular Forces for PH3 YouTube
Step 1 (A) Lewis structure of PH A 3 View the full answer Step 2 Unlock Answer Unlock Previous question Next question Transcribed image text: Part A Draw the Lewis structure of PH3 To add lone pairs, click the button before clicking on the molecule.

Ph3 Lewis Structure Shape
Lewis Structure is the pictorial representation of the arrangement of atoms and valence electrons in the molecule. To know the Lewis Structure, we first know the central atom and the arrangement of other atoms. Here for PH3, the phosphorus atom will take the central position as Hydrogen atoms cannot take a central position in the Lewis Structure.

H3p Lewis Structure
In the PH 3 Lewis structure, there are three single bonds around the phosphorus atom, with three hydrogen atoms attached to it, and on the phosphorus atom, there is one lone pair. PH3 Lewis Structure - How to Draw the Lewis Structure for PH3 Watch on Contents Steps #1 Draw a rough sketch of the structure #2 Next, indicate lone pairs on the atoms
PH3 Lewis Structure (Phosphine) YouTube
The Lewis structure for PH 3 is similar to NH 3. In the PH 3 Lewis structure (and all Lewis structures) hydrogen goes on the outside. Remember, too, that hydrogen only needs two valence electrons to have a full outer shell. In the Lewis structure for PH 3 there are a total of 8 valence electrons. Three pairs will be used in the chemical bonds.